Reverse Osmosis, also known as Ultra-Filtration, represents state-of-the-art technology in water treatment. Reverse Osmosis (RO) was developed in the late 1950's under U.S. Government funding, as a method of desalinating sea water. Today, reverse osmosis has earned its name as the most convenient and thorough method to filter water. It is used by most water bottling plants, and by many industries that require ultra-refined water in manufacturing. This advanced technology is also available to homes and offices for drinking water filtration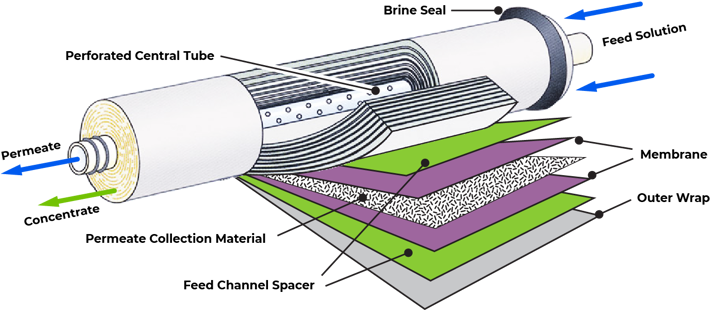
To understand "reverse osmosis," it is probably best to start with normal osmosis. According to Merriam-Webster's Collegiate Dictionary, osmosis is the "movement of a solvent through a semipermeable membrane (as of a living cell) into a solution of higher solute concentration that tends to equalize the concentrations of solute on the two sides of the membrane. A semipermeable membrane is a membrane that will pass some atoms or molecules but not others. Saran™ wrap is a membrane, but it is impermeable to almost everything we commonly throw at it. The best common example of a semipermeable membrane would be the lining of your intestines, or a cell wall. GORE-TEX® is another common semipermeable membrane. GORE-TEX® fabric contains an extremely thin plastic film into which billions of small pores have been cut. The pores are big enough to let water vapor through, but small enough to prevent liquid water from passing.
In the figure above, the membrane allows passage of water molecules but not salt molecules. One way to understand osmotic pressure would be to think of the water molecules on both sides of the membrane. They are in constant motion. On the salty side, some of the pores get plugged with salt atoms, but on the pure-water side that does not happen. Therefore, more water passes from the pure-water side to the salty side, as there are more pores on the pure-water side for the water molecules to pass through. The water on the salty side rises until one of two things occurs:
- The salt concentration becomes the same on both sides of the membrane (which isn't going to happen in this case since there is pure water on one side and salty water on the other).
- The water pressure rises as the height of the column of salty water rises, until it is equal to the osmotic pressure. At that point, osmosis will stop.
In reverse osmosis, the idea is to use the membrane to act like an extremely fine filter to create drinkable water from salty (or otherwise contaminated) water. The salty water is put on one side of the membrane and pressure is applied to stop, and then reverse, the osmotic process. It generally takes a lot of pressure and is fairly slow, but it works.
Reverse osmosis is an extremely effective technology in removing contaminants from your drinking water. The following chart outlines some of RO's capabilities regarding specific contaminants:
In sizing an RO system one must realize that the information regarding a systems performance as stated by the manufacturer, will NOT be what is actually realized when the system is installed in your home. Let's work through an example to find the actual output of an RO system under the following conditions:
TDS = 200 ppm
Water Temperature = 5°C (41°F)
Incoming Pressure = 45 psi
Stated Membrane Output = 50 gpd
The output of all RO systems are tested at 25°C (77°F) and at a specific pressure which can vary from manufacturer to manufacturer (in this case LUMINOR will assume a FilmTech membrane which has a Rated System Pressure of 50 psi). All three of these parameters (TDS, temperature & pressure) will affect the amount of water produced by the RO. To answer this question we must first calculate the Temperature Correction Factor (TFC) maximum daily production (in GPD) of the membrane. To do this, we need to refer to the chart below and select the TFC for a thin film membrane at 41°F, which is 2.58. Now we take the Rated Pressure (Pr) and divide this by the TFC and get 17.4 (45/2.58=17.4) Therefore, with these given water conditions, the TFC Adjusted Flow (maximum production of the membrane) is now 17.4 GPD, simply to account for the cold water.
Now we'll look at the pressure side, but first we have to account for the TDS. For RO to function, a minimum pressure of 1 psi is required for every 100 ppm of TDS. In this case, 15 psi of pressure is needed to force a single drop of water through the membrane. This is known as Osmotic Pressure (Π) and is calculated by the equation (Π = TDS/100). In this case, the Osmotic Pressure is 20 psi. Next we need to calculate the Net Pressure Rating (Pr) which is the Rated System Pressure (Ps) minus the Osmotic Pressure (Pr = Ps - Π). In this case the Net Pressure Rating is 30 psi (50-20). We also need to calculate the Net Operating Pressure (Pn) which is the Applied Pressure (Pa) minus the Osmotic Pressure (Pn = Pa - Π). In this case the Net Operating Pressure is 25 psi (45-20).
To calculate the amount of Expected Production we need to take the temperature adjusted flow and multiply it by the division of the Net Operating Pressure and the Net Pressure Rating (TFC Adjusted Flow X (Pn/Pr)). In this case, the net Expected Flow would be a total of 14.5 GPD (17.4 X (25/30)). So under these conditions, the maximum water that can be produce by this system in a 24-hour period is 14.5 gallons ad ultimately you would have to determine if that was enough or not. Bottom line is to be aware that the rated capacity of the system and the actual amount of water that you will get from the system are nowhere close.
TEMPERATURE CORRECTION FACTORS (TCF)
There are many situations that can lead to fouling of a reverse osmosis membrane:
| Suspended Solids | Calcium Sulfate |
| Iron | Organic Matter |
| Manganese | Microbiological Fouling |
| Silica | Oxidation |
| Colloidal Matter | Hydrolysis |
| Aluminum | Concentration Polarization |
The technology of reverse osmosis can be used in a myriad of applications for both home and industry. The following are just a few of the examples:
| Drinking Water | Food Preparation | Cooking |
| Phtography | Car Wash | Hatcheries |
| Restaurants | Ice Making | Greenhouses |
| Boiler Water | Laboratories | Kidney Dialysis |
| Rinse Waters | Pharmaceutical | Semi-Conductor |
| Metal Plating | Pretreatment | Post Treatment |
| Bio-Medical | Battery Water | Humidification |
| Aquariums | Steam Irons | Plants |
| Baby Formula | Spotless Window Washing | Hemodialysis |
| Cosmetics | Low Sodium Diets | Weight Loss Diets |
| Maple Syrup Production | Wastewater Treatment | Coffee, Tea & other Beverages |
 Skip to main content
Skip to main content

